Online Guest lecture on celebration on “3rd National current Good Manufacturing Practice Day”.
October 10, 2025 2025-10-17 8:38Online Guest lecture on celebration on “3rd National current Good Manufacturing Practice Day”.
Online Guest lecture on celebration on “3rd National current Good Manufacturing Practice Day”.
Name of Department: Faculty of Pharmacy in collaboration with IIC and IQAC
Organizer: Faculty of Pharmacy in collaboration with IIC and IQAC
Activity Incharge: Ms. Shivani Kumar
Date of Activity: 10.10.2025
Time: 11:30 am onwards
Aim or Objective:
- To gain knowledge about the concept, scope, and importance of cGMP in the pharmaceutical industry.
- To understand the regulatory guidelines provided by agencies such as USFDA, WHO, and EMA for CGMP compliance.
- To study the key components of cGMP including personnel, premises, equipment, documentation, and quality control.
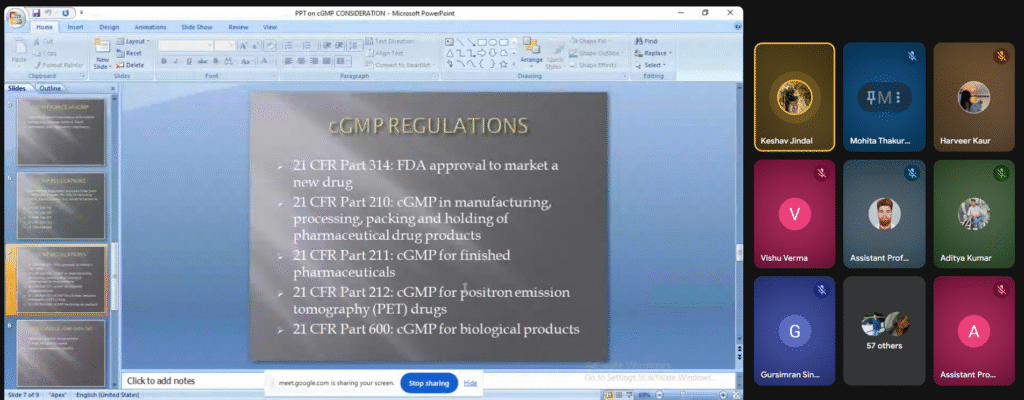
Report:
On the occasion of celebration of 3rd National current Good Manufacturing Practice Day Placebo Club, School of Pharmacy, S. Lal Singh Memorial College of Pharmacy and Mata Jarnail K College of Pharmacy in collaboration with IIC and IQAC, Desh Bhagat University, Mandi Gobindgarh organized an online guest lecture on 10th October 2025. Dr. Keshav Jindal, Professor (Pharmaceutics), Adesh Institute of Pharmacy and Biomedical Science, Adesh University, Bathinda was the speaker.
The speaker was welcomed digitally by Ms. Shivani Kumari, Assistant Professor, School of Pharmacy. The speaker focused the importance of current Good Manufacturing Practice. Quality by design, risk management and assessment, critical process parameters, critical quality attributes, critical quality management were well explained by the speaker. He also gave the knowledge about mini tab and optimization techniques.
There was an interactive question-answers session between the speaker and students at the end of the talk.
All the faculty members and students of Faculty of Pharmacy learnt a lot from this excellent lecture. It was such a wonderful and knowledgeable lecture. Moreover students were made aware about current Good Manufacturing Practice and its importance in Pharmaceutical Industry.
Dr. Puja Gulati ended the session with a vote of thanks to the eminent speaker.
Outcomes:
- Participants gained a comprehensive understanding of the importance and implementation of Current Good Manufacturing Practices in the pharmaceutical industry.
- Students became familiar with global regulatory standards such as WHO, USFDA, and EMA guidelines.
- The session enhanced awareness about quality assurance, documentation, and process validation in drug manufacturing.
- Learners developed insight into practical challenges and solutions in maintaining product quality and safety.
- The expert talk motivated participants to incorporate cGMP principles in their academic projects and future professional practices.







